
During the past two years, society has looked to the pharmaceutical sector to get us out of the COVID-pandemic. Around the world, people are dependent on pharmaceuticals to overcome diseases every day.
The necessity for pharmaceutical products has meant that historically, regulation has prioritised ensuring product quality and consumer safety over environmental sustainability. However, it is becoming increasingly apparent that the sector has some significant sustainability challenges to deal with. Take for example pollution from synthetic chemicals (incl. plastics and pharmaceuticals), which currently exceeds our planetary boundaries – or, in other words, the safe operating space for humanity. To tackle for instance the issue of wastewater pollution from drug manufacturing and pharmaceuticals ending up in the environment from production and after use, better screening of chemicals to determine the impact on ecosystems is needed. Important to remember is also, that the pharma companies themselves must play their part in battling the conventional environmental issues related to reducing waste, water use, and emissions.
The adverse impacts on human health and ecosystems in the pharma value chain constitute both reputational and business continuity risks, further emphasising the need for pharma companies to start working systematically with sustainability. While we do see that increased investor sentiment, expected upcoming regulation, and a greater desire to reduce negative impact are making an increasing number of pharma companies move ahead on sustainability and set ambitious targets, it is also clear that the sector as a whole needs to ramp up their environmental ambitions. With this article, we aim to point to where environmental hot spots typically lie within the pharma value chain and suggest an approach for companies to start addressing these issues.
Sustainability challenges in the European pharma sector
The major impact areas in the European pharma sector’s value chain are supplier production, logistics, operations, and after-use. It should be noted, however, that this list is non-exhaustive, and that individual companies may face their own specific challenges.

Drivers for change: Regulation
Pharma is a highly regulated sector when it comes to product safety, but regulation on environmental sustainability is lacking – a fact acknowledged by both industry players and regulators. On the bright side, the EU Commission (EC) seems to be turning the tides with the publishing of their first Pharmaceuticals Strategy for Europe under the European Green Deal. As part of this, the EC is currently reviewing pharma legislation to build on past frameworks and environmental risk assessments. Additionally, the EC has adopted a new Chemicals Strategy which aims towards creating a toxic-free environment. This strategy will also target pharmaceuticals, notably through amendments to REACH (list of substances of Very High Concern)17. In the light of these developments, being at the forefront of understanding the environmental impacts of products will be a significant advantage for pharma companies.
Drivers for change: Frontrunners
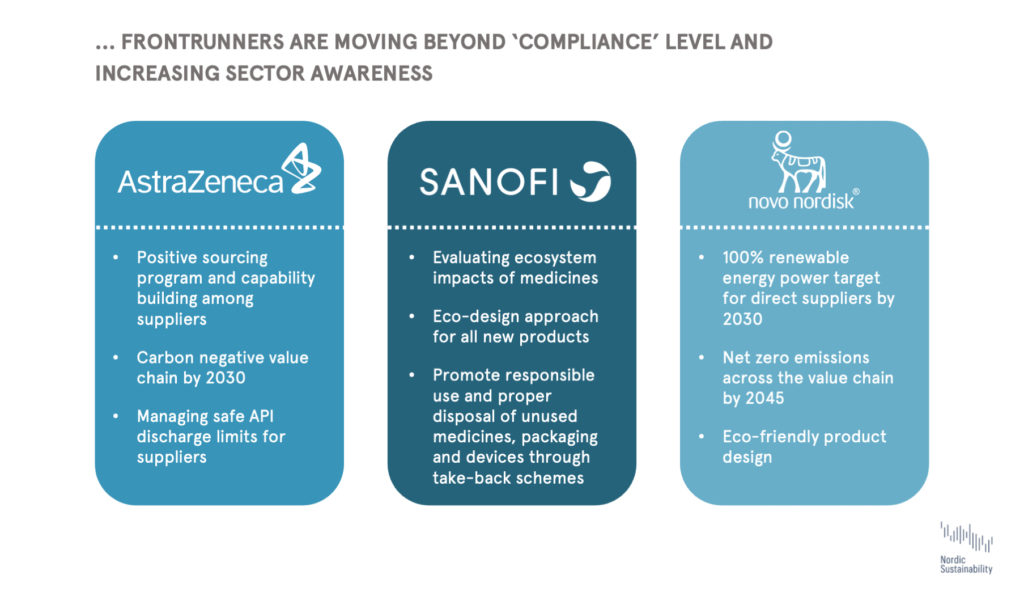
Promisingly, we are seeing leading pharma companies launch net zero strategies and address after-use impacts of medicines. These frontrunners are moving beyond ‘compliance’ and going above current regulatory requirements, driving the sustainability movement in the sector as other companies are trying to catch up. For instance, AstraZeneca and Sanofi have begun to apply safe API discharge limits for suppliers, and have dedicated research on the API issue. We also see pharma companies like Novo Nordisk and Sanofi launching ‘eco-design’ strategies to address the entire life cycle of products. While the lack of regulation and transparency on the matter creates uncertainty around what can be considered ‘eco’, we can expect green chemistry to become an important field of innovation going forward. Lastly, we also see firms like Novo Nordisk working with suppliers, for instance by requiring them to set ambitious climate targets; thereby reducing their upstream and downstream footprint.
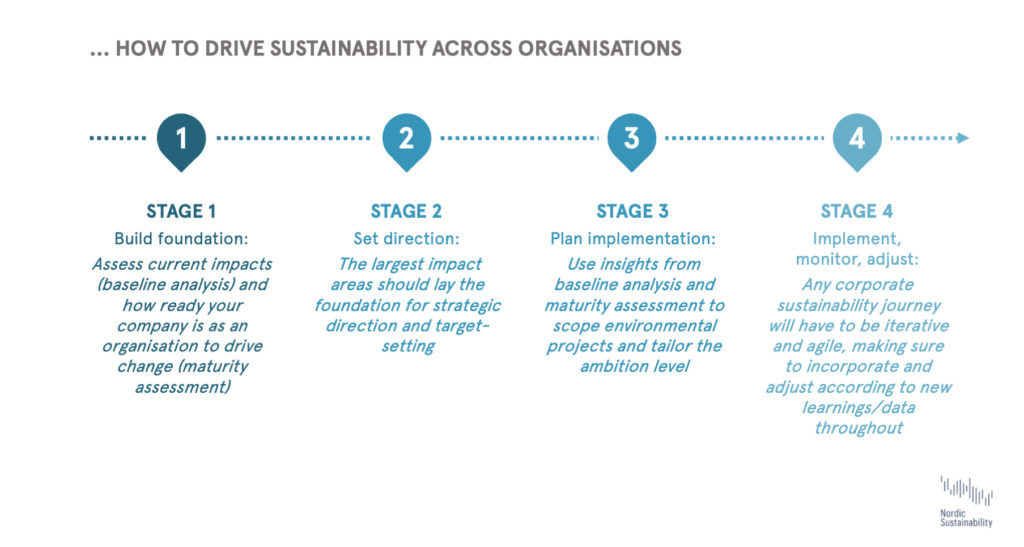
How to get started – the corporate sustainability journey
Tackling the above impact areas along the pharma value chain is not looking that different from the usual corporate sustainability journey. Using existing resources from recognized organisations such as the Science Based Targets initiative to set climate goals will help companies understand what it means to be truly sustainable, while peers’ progress on sustainability should be considered when determining the ambition level. Keeping in mind company-specific challenges and resources, we can divide the journey into the following four steps:
The urgency for pharma to embed sustainability into its strategic core is clear – both to stay competitive as frontrunners, as well as to minimise business risks while serving a wider societal impact beyond the purpose of producing pharmaceuticals, namely the environmental sustainability of our planet.
If you have any questions regarding the content of this article, do not hesitate to reach out to Maja Johannessen, Manager, on mjo@nordicsustainability.com
Want to know more?
If you have any questions regarding the content of this article, do not hesitate to reach out to Maja Johannessen, Manager, on mjo@nordicsustainability.com



